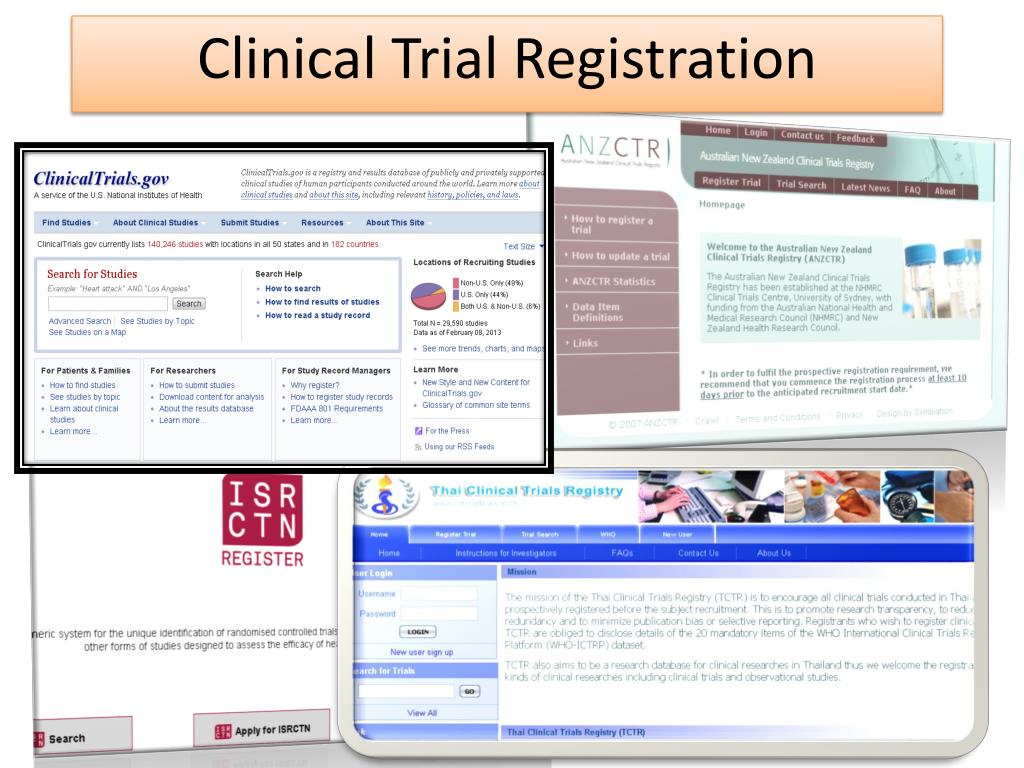
Clinical Study Online Registration - To register a trial, submit the details directly to any one of the primary registries in the who registry network or an icmje approved. A structured online system, such as the clinicaltrials.gov results database, that provides the public with access to registration and. You can access the registration site directly at: When is registration and reporting required? For additional information on the registration. Clinicaltrials.gov registration is required for all federally sponsored clinical trials or studies. Als.gov registration guide overview clinicaltrials.gov is a publicly available registry and results database of federally and.
To register a trial, submit the details directly to any one of the primary registries in the who registry network or an icmje approved. Als.gov registration guide overview clinicaltrials.gov is a publicly available registry and results database of federally and. For additional information on the registration. Clinicaltrials.gov registration is required for all federally sponsored clinical trials or studies. When is registration and reporting required? You can access the registration site directly at: A structured online system, such as the clinicaltrials.gov results database, that provides the public with access to registration and.
You can access the registration site directly at: To register a trial, submit the details directly to any one of the primary registries in the who registry network or an icmje approved. Clinicaltrials.gov registration is required for all federally sponsored clinical trials or studies. A structured online system, such as the clinicaltrials.gov results database, that provides the public with access to registration and. For additional information on the registration. When is registration and reporting required? Als.gov registration guide overview clinicaltrials.gov is a publicly available registry and results database of federally and.
How to register a research trial in Clinical Trials Registry of India
A structured online system, such as the clinicaltrials.gov results database, that provides the public with access to registration and. Clinicaltrials.gov registration is required for all federally sponsored clinical trials or studies. When is registration and reporting required? To register a trial, submit the details directly to any one of the primary registries in the who registry network or an icmje.
Serbia Clinical Trial Registration Guideline Regulamedica
To register a trial, submit the details directly to any one of the primary registries in the who registry network or an icmje approved. When is registration and reporting required? For additional information on the registration. A structured online system, such as the clinicaltrials.gov results database, that provides the public with access to registration and. Als.gov registration guide overview clinicaltrials.gov.
Clinical Trial Online Courses JLI Blog
Als.gov registration guide overview clinicaltrials.gov is a publicly available registry and results database of federally and. You can access the registration site directly at: When is registration and reporting required? For additional information on the registration. A structured online system, such as the clinicaltrials.gov results database, that provides the public with access to registration and.
How to Register Clinical The Clinical Establishments Registration
Clinicaltrials.gov registration is required for all federally sponsored clinical trials or studies. You can access the registration site directly at: When is registration and reporting required? A structured online system, such as the clinicaltrials.gov results database, that provides the public with access to registration and. For additional information on the registration.
How to register your clinical trials and studies at the ISRCTN registry
Als.gov registration guide overview clinicaltrials.gov is a publicly available registry and results database of federally and. To register a trial, submit the details directly to any one of the primary registries in the who registry network or an icmje approved. A structured online system, such as the clinicaltrials.gov results database, that provides the public with access to registration and. Clinicaltrials.gov.
PPT Clinical Trial Registration PowerPoint Presentation, free
To register a trial, submit the details directly to any one of the primary registries in the who registry network or an icmje approved. Clinicaltrials.gov registration is required for all federally sponsored clinical trials or studies. For additional information on the registration. When is registration and reporting required? Als.gov registration guide overview clinicaltrials.gov is a publicly available registry and results.
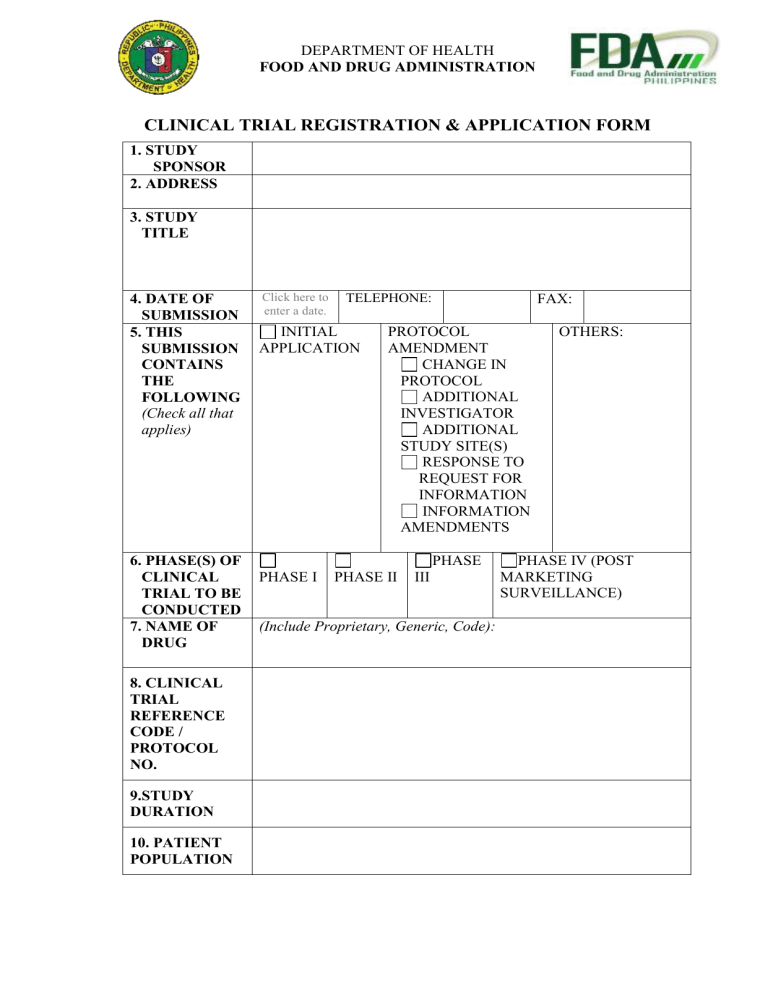
Clinical Trial Registration Application Form
To register a trial, submit the details directly to any one of the primary registries in the who registry network or an icmje approved. A structured online system, such as the clinicaltrials.gov results database, that provides the public with access to registration and. For additional information on the registration. You can access the registration site directly at: Clinicaltrials.gov registration is.
UK launches new system to achieve 100 clinical trial registration
To register a trial, submit the details directly to any one of the primary registries in the who registry network or an icmje approved. For additional information on the registration. You can access the registration site directly at: A structured online system, such as the clinicaltrials.gov results database, that provides the public with access to registration and. When is registration.
A Modernized ClinicalTrials.gov Website is Coming NCBI Insights
You can access the registration site directly at: To register a trial, submit the details directly to any one of the primary registries in the who registry network or an icmje approved. Clinicaltrials.gov registration is required for all federally sponsored clinical trials or studies. When is registration and reporting required? Als.gov registration guide overview clinicaltrials.gov is a publicly available registry.
Fillable Online clinicaldepartments musc Registration Form Clinical
Als.gov registration guide overview clinicaltrials.gov is a publicly available registry and results database of federally and. For additional information on the registration. To register a trial, submit the details directly to any one of the primary registries in the who registry network or an icmje approved. You can access the registration site directly at: A structured online system, such as.
When Is Registration And Reporting Required?
For additional information on the registration. A structured online system, such as the clinicaltrials.gov results database, that provides the public with access to registration and. Clinicaltrials.gov registration is required for all federally sponsored clinical trials or studies. To register a trial, submit the details directly to any one of the primary registries in the who registry network or an icmje approved.
You Can Access The Registration Site Directly At:
Als.gov registration guide overview clinicaltrials.gov is a publicly available registry and results database of federally and.